Clean area control
1. Temperature and Humidity
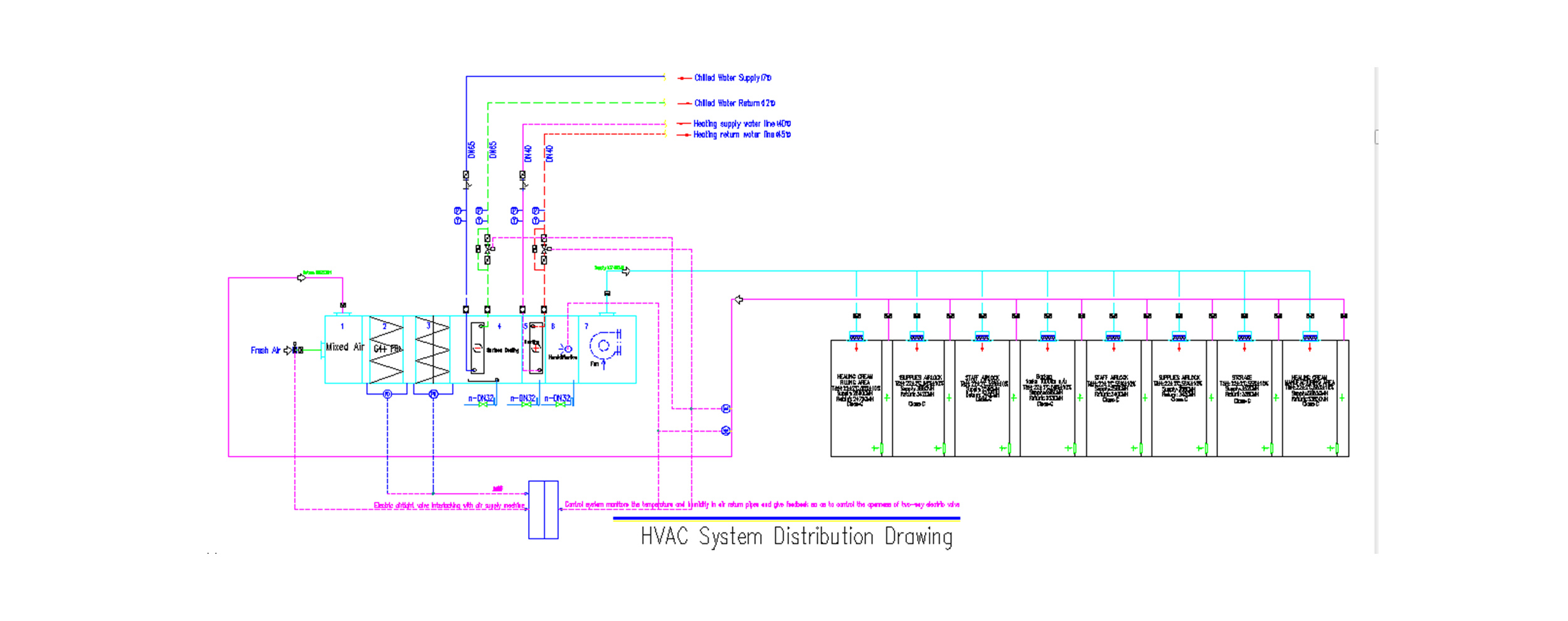
The pharmaceutical production quality management standard 6 (revised in 1998) pointed out: The temperature and humidity of the clean room (area) should be compatible with the pharmaceutical production process requirements. When there is no special requirement, the temperature should be controlled at 18^26℃, and the relative humidity should be controlled at 45%~65%. In the actual design, the temperature and humidity of the clean room (area) depend on the following three aspects: the production process requirements of medicines, human comfort and outdoor environmental conditions. First of all, the production process requirements of medicines should be guaranteed. For example, the humidity of the solid preparation workshop is controlled at 45% to 60%, and the powder injection is controlled at about 45%. Otherwise, the medicine is easy to absorb moisture and affect the quality; exceptions are made for special medicines, and the injection water injection has no special requirements for humidity , The main consideration is human comfort, the southern control is slightly higher, the northern is slightly lower.
2. Personnel purification
Experimental data shows that people are the main source of pollution in clean rooms (areas), and people entering the clean room (areas) must be cleaned: take off shoes and clothing, wash bare parts of the body, and change to clean clothes that meet the requirements.
3. Material purification
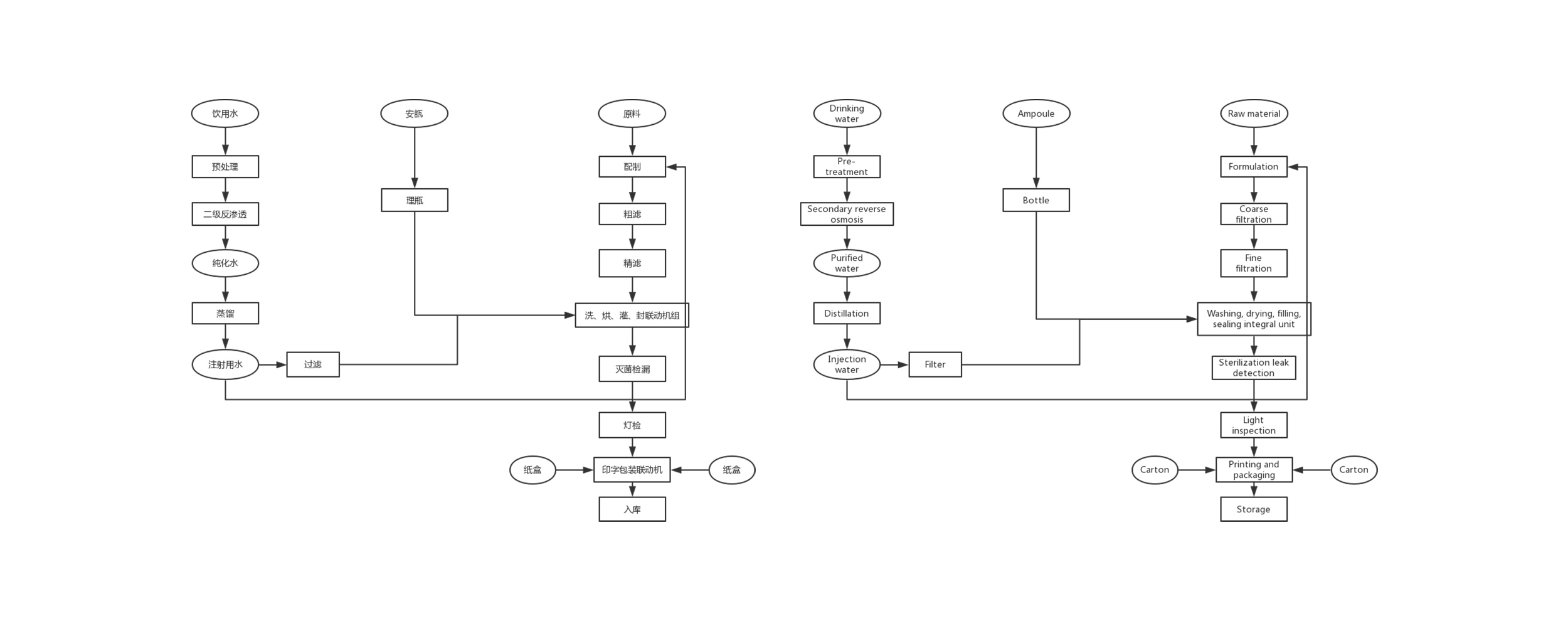
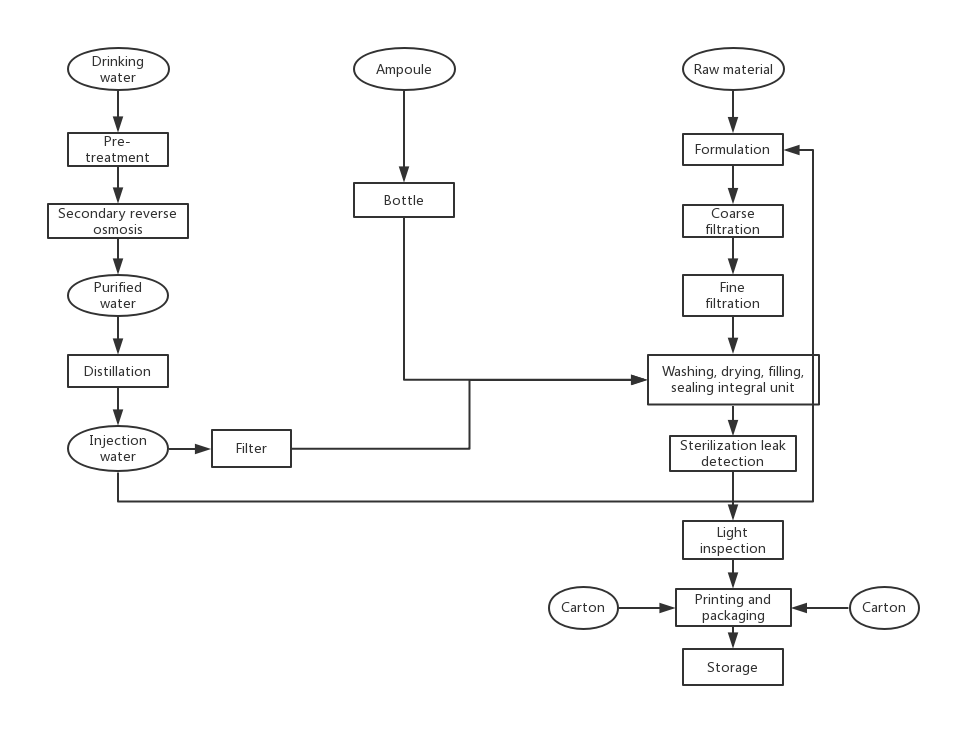
Before the material enters the clean area, it is necessary to perform the necessary treatment on the outer packaging in the outer clean room, clean and peel off the outer packaging. For those who cannot remove the outer packaging, they should be cleaned or wiped to ensure that the surface is clean, and then enter the clean area through the buffer room. Small objects can use transfer windows with anti-pollution facilities, such as transfer windows with UV lamps or high-efficiency filters inside.
There is a certain supply and return air in the buffer room, which can play the role of replacing the clean air flow, and at the same time maintain a certain pressure difference with the clean room (area) and the non-clean room, which can effectively prevent cross-contamination. There must be more than two doors in the buffer room, and interlocking measures to prevent simultaneous opening.
4. Air purification and cleanliness level
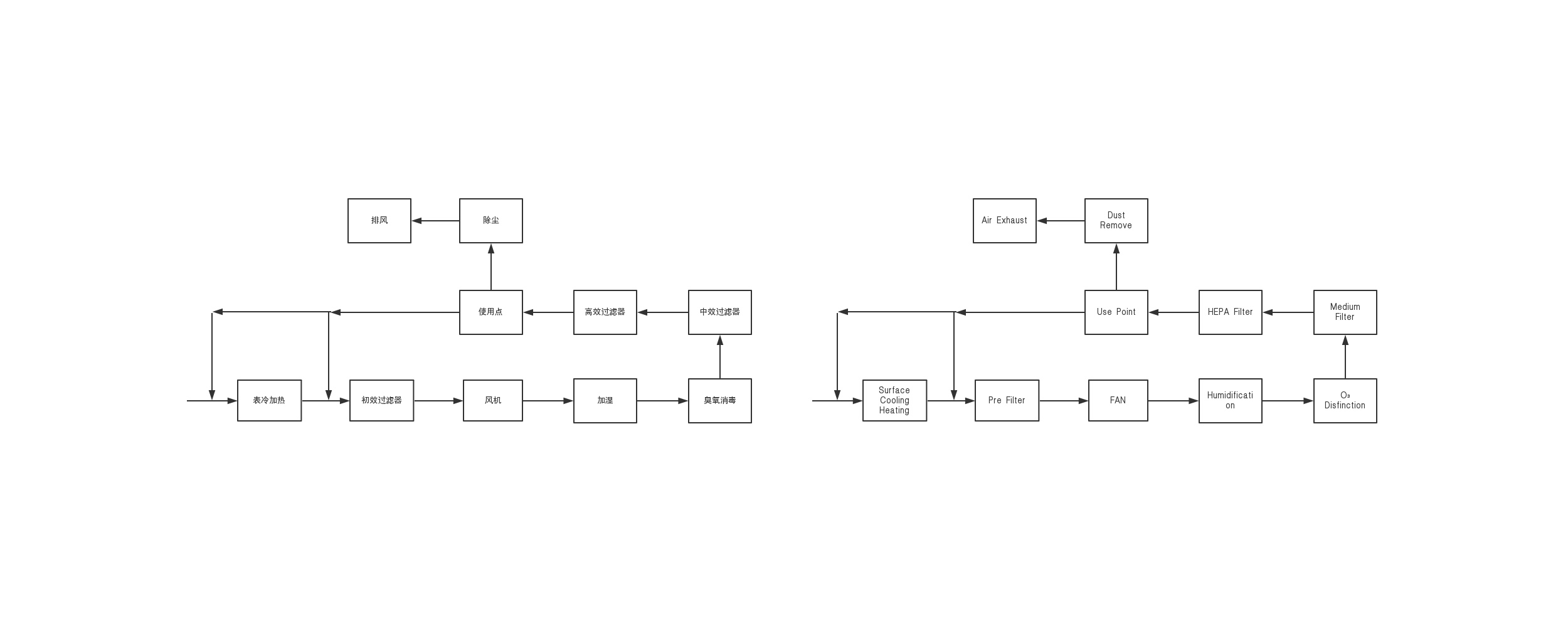
Air purification usually uses primary, intermediate and high efficiency three-stage filtration. The clean air in the clean room (area) can be recycled, but the clean room that produces dust, such as raw material weighing room, charcoal washing room, etc., is generally re-used after the dust removal treatment meets the requirements of clean air, but it is clean for large dust production The room is generally discharged directly after meeting the requirements of environmental protection through dust removal.
The cleanliness level of the clean room (area) is determined according to different pharmaceutical dosage forms, so as to determine the times of sending and returning of clean air. GMP has strict requirements for the cleanliness level of each dosage form, and the final cleanliness level of the injection water workshop that is not sterilized is 7 levels. Relative and filling levels are higher at level 7, while raw material storage, weighing and concentration levels are lower at level 8.